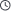In high-calcium/magnesium ion (Ca²⁺/Mg²⁺) or high-sulfate (SO₄²⁻) environments, the scale inhibition mechanism of SMAS (Sulfonated Maleic Anhydride Copolymer) is achieved through the following synergistic effects:
1. Chelation
- The carboxylate groups (–COO⁻) preferentially bind with Ca²⁺/Mg²⁺, forming soluble complexes that reduce the concentration of free ions, thereby delaying the nucleation of scales such as CaCO₃, CaSO₄, or Mg(OH)₂.
- Even in high-ion-concentration environments, SMAS’s multiple carboxylate sites can partially chelate ions, inhibiting precipitation in supersaturated solutions (threshold effect).
2. Crystal Distortion
- The –SO₃⁻ and –COO⁻ groups of SMAS adsorb onto the surfaces of microcrystals, disrupting the growth orientation of CaCO₃ (calcite) or CaSO₄ (gypsum), leading to the formation of loose, amorphous, and non-adherent scale layers that are easily flushed away by water flow.
- In Mg²⁺ systems, it inhibits the formation of flocculent Mg(OH)₂ precipitates.
3. Electrostatic Repulsion
- The sulfonate groups (–SO₃⁻) remain highly ionized even in high-ionic-strength environments. By adsorbing onto particle or crystal nuclei surfaces, they create negative charge repulsion (reducing Zeta potential), preventing particle aggregation or deposition.
- Particularly effective for dispersing sulfate scales such as CaSO₄ or BaSO₄.
4. Dispersion
- The long polymer chains of SMAS envelop microcrystals or suspended particles, creating steric hindrance that prevents their deposition onto pipe walls or membrane surfaces.
Adaptability in High-Ion Environments
- Salt Resistance: The strong hydration capability of –SO₃⁻ prevents SMAS from salting out in high-SO₄²⁻/Cl⁻ solutions.
- pH Stability: The –COO⁻/–SO₃⁻ groups remain effectively ionized under both alkaline (e.g., cooling water) and acidic (e.g., reverse osmosis) conditions.
Application Scenarios
- Oilfield reinjection water (high Ca²⁺/SO₄²⁻), seawater desalination (high Mg²⁺), industrial cooling water (high hardness + high alkalinity).
Through its chelation-distortion-dispersion-electrostatic stabilization multi-mechanism, SMAS maintains high scale inhibition efficiency even in harsh water conditions, outperforming single-functional-group polymers (e.g., polyacrylic acid, PAA). Its molecular weight and sulfonation degree should be optimized based on specific water quality requirements.