It’s important to clarify that SMAS is rarely used alone. Its primary function is as a monomer incorporated into polymers (e.g., copolymerized with acrylamide, acrylic acid) that are then used as fluid loss additives. The mechanism described below is for these SMAS-containing copolymers.
These copolymers reduce fluid loss through a combination of two key mechanisms: Adsorption and Hydration, which work together to form a low-permeability filter cake.
1. Adsorption: Anchoring to the Surface
The first step is for the polymer chain to attach itself to the solid particles in the drilling mud (e.g., clay, barite).
- Mechanism: The SMAS unit in the copolymer provides a strongly anionic sulfonate group (-SO₃⁻). The surfaces of most drilling mud solids (like clays) carry a slight negative charge but have positively charged edges and sites. The anionic sulfonate group has a very high affinity for these positive sites on the particle surfaces.
- Result: The polymer chain adsorbs onto the solid particles, effectively “anchoring” itself. This adsorption is particularly strong and resistant to displacement due to the persistent anionic charge of the sulfonate group, even in the high ionic strength environments of drilling muds.
2. Hydration: Building the Protective Layer
Once adsorbed, the polymer chain extends into the water phase and hydrates.
- Mechanism: The sulfonate group is extremely hydrophilic (water-loving). It attracts and binds a large number of water molecules, forming a massive hydration shell around itself and the entire polymer chain.
- Result: The adsorbed polymer chain swells into a large, hydrated, and viscous structure. This creates a physical barrier that:
- Restricts Pore Throats: The swollen polymer molecules effectively narrow the passages between solid particles in the forming filter cake.
- Imparts Lubricity: The hydrated layer makes the cake slicker, allowing for the deposition of a thin, dense, and impermeable filter cake.
3. Synergistic Effect: Plugging Pores and Reducing Permeability
The combination of adsorption and hydration is what creates an effective seal.
- Process: As the drilling fluid filtrate attempts to invade the formation, solid particles are deposited on the wellbore wall, forming the filter cake. The SMAS-containing copolymers, already adsorbed onto these particles, simultaneously hydrate.
- Result: The hydrated polymers bridge and plug the microscopic pores between the larger solid particles. This dramatically reduces the permeability of the filter cake, creating a physical barrier that severely limits the further passage of fluid (filtrate) into the formation.
Why SMAS is Particularly Effective for This Role
- Divalent Cation Tolerance: Unlike carboxylate groups (-COO⁻), which precipitate in the presence of calcium or magnesium ions, the sulfonate group from SMAS is highly stable. This allows the polymer to remain soluble, adsorb, and hydrate effectively even in hard water and high-salinity environments, which are common in drilling operations.
- Thermal Stability: The C-S bond in the sulfonate group is very stable, allowing polymers containing SMAS to perform their fluid loss control function effectively at higher downhole temperatures.
Summary of the Process
The following flowchart illustrates the sequential mechanism of how SMAS-containing copolymers reduce fluid loss:
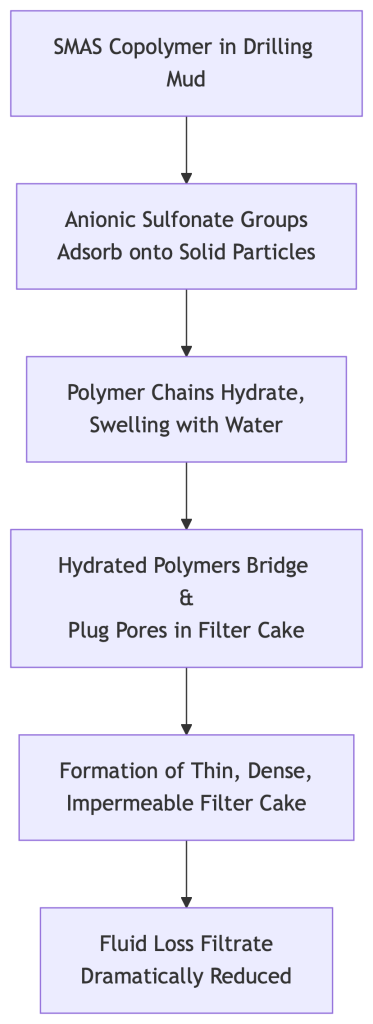
In essence, SMAS-based fluid loss additives work by first anchoring themselves to mud solids and then swelling with water to physically plug the pores in the filter cake, forming an effective barrier that minimizes fluid loss into the formation. Their key advantage is the ability to perform this function reliably in the harsh conditions of high salinity and temperature.






