Here’s a detailed overview of techniques for treating wastewater containing sodium bromate (NaBrO₃), covering both its degradation and resource recovery, based on current industrial practices and research.
Sodium bromate (NaBrO₃) is a strong oxidizing agent often found in wastewater from industries like textiles (denim dyeing), pharmaceuticals, and chemical synthesis. Its treatment is crucial due to its toxicity, carcinogenicity, and potential environmental persistence. Treatment strategies often aim to either destroy the bromate ion (BrO₃⁻) or recover valuable resources like bromine (Br₂) or bromide (Br⁻).
1. Source Reduction and Pre-Treatment
The best approach is to minimize bromate generation at the source.
- In Water Treatment: If bromate forms during ozonation of bromide-containing water, optimize ozone dose, lower pH, or use ammonia to minimize its formation.
- In Industrial Processes: Substitute sodium bromate with safer oxidants like hydrogen peroxide (H₂O₂) or sodium persulfate where technically feasible, especially in cosmetics or textiles.
2. Degradation and Destruction Techniques
These methods focus on converting bromate (BrO₃⁻) into less harmful bromide (Br⁻) or inert compounds.
2.1. Chemical Reduction
This is a common and effective method where a reducing agent donates electrons to bromate, reducing it to bromide.
- Reducing Agents:
- Ferrous Iron (Fe²⁺): Often used as FeSO₄. Fe²⁺ reduces BrO₃⁻ to Br⁻. The reaction is faster at low pH (acidic conditions).
- Reaction:
BrO₃⁻ + 6Fe²⁺ + 6H⁺ → Br⁻ + 6Fe³⁺ + 3H₂O
- Reaction:
- Zero-Valent Iron (ZVI): Fine iron powder acts as both a source of Fe²⁺ and an electron donor. It’s effective over a wider pH range and can be used in permeable reactive barriers.
- Sulfite Compounds (e.g., Sodium Sulfite – Na₂SO₃): Can reduce bromate, but kinetics may be slower without catalysts.
- Ferrous Iron (Fe²⁺): Often used as FeSO₄. Fe²⁺ reduces BrO₃⁻ to Br⁻. The reaction is faster at low pH (acidic conditions).
- Pros: High efficiency, relatively simple implementation, fast reaction.
- Cons: Can generate significant sludge (from iron hydroxide formation), requires pH adjustment, and adds dissolved ions (e.g., sulfate from FeSO₄) to the water.
2.2. Advanced Reduction Processes (ARPs)
ARPs combine reducing agents with ultraviolet (UV) irradiation to generate highly reactive reducing radicals.
- UV/Sulfite: UV light activates sulfite ions (SO₃²⁻) to form highly reductive hydrated electrons (eₐq⁻), which efficiently reduce bromate to bromide.
- Reaction:
BrO₃⁻ + 6eₐq⁻ + 6H₂O → Br⁻ + 9OH⁻
- Reaction:
- Pros: Very fast and efficient degradation, minimal chemical sludge.
- Cons: High energy cost for UV lamps, water clarity affects efficiency (turbidity must be low), requires precise control.
2.3. Heterogeneous Catalytic Reduction
This method uses a solid catalyst to enhance reduction.
- Catalysts: Palladium (Pd), titanium dioxide (TiO₂), or activated carbon supported catalysts.
- Process: Hydrogen gas (H₂) is often used as a clean reducing agent in the presence of a Pd catalyst.
- Reaction:
BrO₃⁻ + 3H₂ → Br⁻ + 3H₂O(catalyzed by Pd/Al₂O₃ or similar)
- Reaction:
- Pros: High specificity, no chemical sludge, catalyst can be reusable.
- Cons: Catalyst cost (especially noble metals), risk of catalyst poisoning by other wastewater constituents.
2.4. Biological Reduction
Microorganisms can reduce bromate under specific conditions.
- Process: Certain anaerobic bacteria can use bromate as a terminal electron acceptor for respiration, reducing it to bromide.
- Conditions: Requires anoxic or anaerobic environments, often in constructed wetlands or specialized bioreactors. Organic carbon (e.g., methanol, acetate) is needed as an electron donor.
- Pros: Environmentally friendly, low operational cost, minimal chemical usage.
- Cons: Slow process, requires careful control of environment (pH, temperature, nutrients), susceptible to toxicity from other wastewater components.
2.5. Adsorption
While not a destruction method, adsorption can concentrate bromate for further treatment or disposal.
- Adsorbents: Specific types of activated carbon, ion exchange resins, or metal-doped oxides can remove bromate from water.
- Pros: Simple operation, effective for low concentrations.
- Cons: Adsorbent requires regeneration or disposal, can be a costly interim step, not a final solution.
3. Resource Recovery Techniques
Instead of just destroying bromate, these methods aim to recover valuable bromine compounds, turning waste into a resource. This is often preferred in industrial settings for economic and sustainability reasons.
- General Principle: Bromate (BrO₃⁻) is first reduced to bromide (Br⁻). The bromide-rich stream is then processed to recover bromine.
- Process Flow (Typical):
- Pre-treatment & Concentration: Remove interfering organics and solids via steps like activated carbon adsorption, filtration, or evaporation. This is critical to avoid fouling and side reactions in later stages.
- Reduction to Bromide: Convert BrO₃⁻ to Br⁻ using a suitable method like chemical reduction (e.g., with Fe²⁺).
- Bromine Recovery: The concentrated bromide solution is acidified and oxidized to produce elemental bromine (Br₂), which can be stripped out and collected.
- Acidification and Oxidation: Add acid (e.g., H₂SO₄) and an oxidant like chlorine (Cl₂) or hypochlorite (OCl⁻).
- Reaction:
2Br⁻ + Cl₂ → Br₂ + 2Cl⁻
- Reaction:
- Stripping: Air or steam is blown through the solution to strip out volatile Br₂ gas.
- Absorption: The Br₂-laden gas is passed through an absorption column where it reacts with a base (e.g., soda ash) or reducing agent to form a concentrated bromide/bromate solution or hydrobromic acid.
- Acidification and Oxidation: Add acid (e.g., H₂SO₄) and an oxidant like chlorine (Cl₂) or hypochlorite (OCl⁻).
- Crystallization (Alternative): In some cases, the bromide solution (after step 2) is further purified and evaporated to recover solid sodium bromide (NaBr) for reuse.
The following flowchart outlines the key decision pathways for treating sodium bromate wastewater:
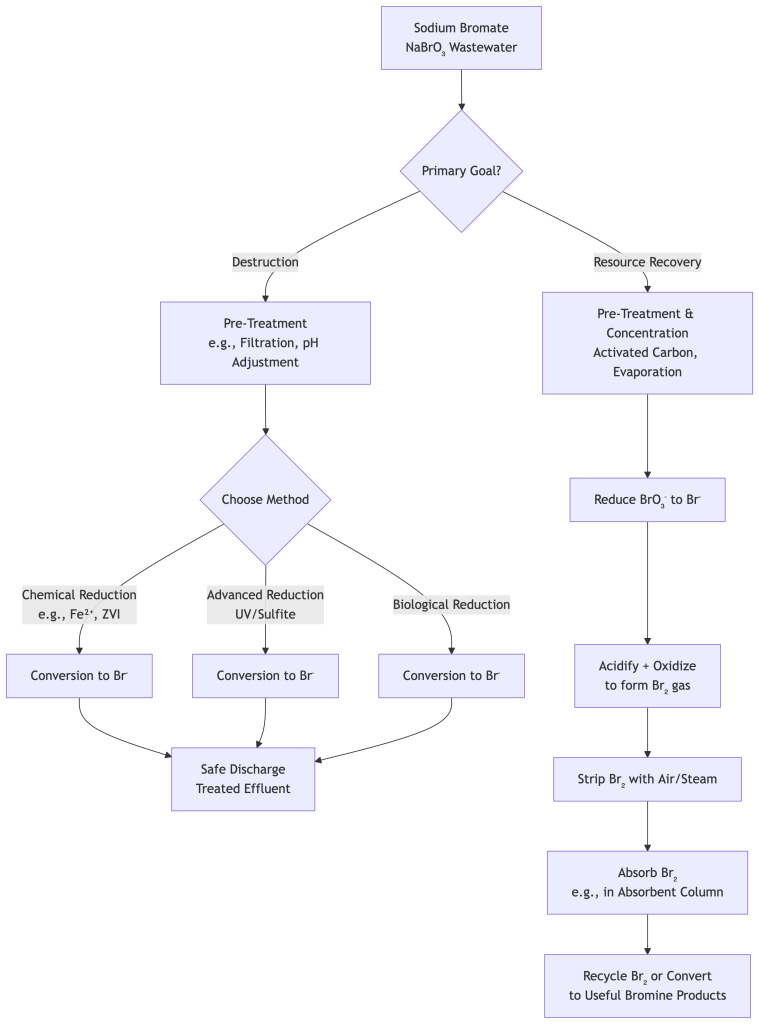
Comparison of Treatment Techniques
| Technique | Mechanism | Pros | Cons | Best For |
|---|---|---|---|---|
| Chemical Reduction | Reduction to Br⁻ using Fe²⁺, ZVI | Simple, effective, fast | Chemical sludge, pH control | Centralized wastewater plants |
| Advanced Reduction (UV/Sulfite) | Radical reduction by eₐq⁻ | Very fast, no sludge | High energy cost, low turbidity | Polishing low-turbidity water |
| Biological Reduction | Microbial respiration | Low cost, eco-friendly | Slow, sensitive to conditions | Large-scale anaerobic digestion |
| Resource Recovery | Reduction to Br⁻, then Br₂ recovery | Economic benefit, sustainable | Complex process, high CAPEX | Industrial streams with high [Br] |
Selection Considerations
Choosing the right technology depends on:
- Bromate Concentration: High concentrations often favor resource recovery for economic return.
- Water Matrix: The presence of other contaminants (organics, salts, solids) can interfere with some treatments and requires pre-treatment.
- Flow Rate and Volume: Biological systems or ARPs suit large volumes, while chemical reduction is adaptable to various scales.
- Cost: Capital expenditure (CAPEX) vs. operational expenditure (OPEX). Recovery systems have high CAPEX but can generate revenue.
- Regulatory Requirements: The required level of bromate removal and final discharge standards dictate the necessary treatment efficiency.
Conclusion
Treating sodium bromate wastewater effectively involves a choice between destruction and resource recovery. For simple destruction, chemical reduction with ferrous iron (Fe²⁺) is a robust and widely applicable method. Where higher efficiency is desired and water clarity allows, advanced reduction processes like UV/sulfite are excellent. For large industrial wastewater streams with significant bromate content, resource recovery to elemental bromine or sodium bromide is the most sustainable and economically attractive option, though it requires more complex process integration.
Always conduct lab-scale treatability tests with the actual wastewater stream to select and optimize the most effective and economical process.






