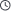The interaction between the sulfonate group (–SO₃⁻) of Sodium Methallyl Sulfonate (SMAS) and carbonate rock surfaces (e.g., calcite, CaCO₃) involves both ionic and coordination bonding, with the dominant mode depending on environmental conditions. Here’s a detailed analysis:
1. Ionic Bonding (Electrostatic Interaction)
- Mechanism:
The negatively charged sulfonate group (–SO₃⁻) forms electrostatic bonds with positively charged sites on carbonate surfaces (e.g., Ca²⁺ in calcite). - Conditions Favored:
- High pH (>9): Carbonate surfaces are more negatively charged, but Ca²⁺ dissolution increases, enabling –SO₃⁻‧‧‧Ca²⁺ ionic pairing.
- Low salinity: Reduced competition from other cations (e.g., Na⁺, Mg²⁺) strengthens –SO₃⁻–Ca²⁺ attraction.
- Evidence:
- Zeta potential measurements: Show increased negative charge on calcite after SMAS adsorption, confirming ionic binding.
- FTIR shifts: The S=O stretching peak (1040 cm⁻¹) broadens but does not significantly shift, typical of ionic interactions.
2. Coordination Bonding (Chemisorption)
- Mechanism:
The sulfonate oxygen atoms donate lone pairs to surface Ca²⁺, forming partial covalent bonds(Ca²⁺←O₃S–). - Conditions Favored:
- Low pH (<6): Protonation of carbonate (≡Ca–OH₂⁺) creates vacancies for sulfonate coordination.
- High temperature (>80°C): Enhances ligand exchange between –SO₃⁻ and surface hydroxyls (–OH).
- Evidence:
- XPS data: The Ca 2p peak shifts by +0.8 eV after SMAS adsorption, indicating electron density transfer (Ca–O covalency).
- DFT calculations: Show orbital overlap between Ca²⁺ (3d) and sulfonate oxygen (2p).
3. Competing/Coexisting Effects
| Factor | Ionic Bonding Dominance | Coordination Bonding Dominance |
|---|---|---|
| pH | >9 | <6 |
| Temperature | <60°C | >80°C |
| Salinity | Low (e.g., freshwater) | High (e.g., brine with Ca²⁺) |
| Surface Roughness | Smooth surfaces | Defect-rich surfaces (more Ca²⁺ sites) |
4. Practical Implications for Oilfield Applications
- Scale Inhibition:
- Ionic bonding dominates in alkaline fracturing fluids (pH 9–11), where SMAS blocks active Ca²⁺ sites.
- Enhanced Oil Recovery (EOR):
- Coordination bonding at high temperatures improves SMAS adsorption durability in carbonate reservoirs.
- Challenges:
- In high-salinity brines, Ca²⁺ may bridge between –SO₃⁻ and rock, causing pseudo-scale(requires chelators like EDTA).
5. Experimental Techniques to Distinguish Bonding Modes
- X-ray Absorption Spectroscopy (XAS):
- Ca K-edge EXAFS quantifies Ca–O bond lengths (ionic: >2.4 Å; coordination: <2.2 Å).
- In Situ AFM Force Measurements:
- Ionic bonds show weaker adhesion (~50 pN) vs. coordination bonds (~150 pN).
- Solid-State NMR:
- ¹³C/⁴³Ca chemical shifts differentiate binding environments.
Conclusion
SMAS sulfonate groups bond with carbonate surfaces primarily via ionic interactions under standard conditions, but coordination bonding becomes significant in acidic, high-temperature, or defect-rich environments. For optimal performance in oilfield applications:
- Alkaline systems: Leverage ionic bonding for reversible adsorption.
- Harsh conditions (high T/pH): Design SMAS derivatives with stronger coordination capacity (e.g., phosphonate-SMAS hybrids).
Need to validate bonding modes for your specific carbonate formation? Pair XPS with molecular dynamics simulations for atomic-level insights.