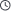In the molecule of Sodium Methallyl Sulfonate (SMAS), the two key functional groups that determine its core chemical properties and application value are the sodium sulfonate group (-SO₃Na) and the carbon-carbon double bond (C=C, located in the methallyl structural unit CH₂=C(CH₃)-). These two groups respectively support SMAS’s core applications in oilfields, polymer synthesis, and other fields from the two dimensions of “solubility/stability” and “reactivity/functionalization”. Their specific mechanisms of action are as follows:
1. Sodium Sulfonate Group (-SO₃Na): Determines “Hydrophilicity, Salt Resistance, and High Charge Density”
The sodium sulfonate group is a strongly polar ionic functional group in the SMAS molecule, which directly endows SMAS with the core chemical properties of “easy solubility, salt stability, and charge-carrying”—this is the foundation for its adaptability to aqueous systems (such as oilfield water-based drilling fluids and aqueous polymerization reactions):
- Strong Hydrophilicity and Water Solubility: The sulfonate group (-SO₃⁻) in -SO₃Na carries a negative charge and forms stable ionic bonds with Na⁺, which can dissociate quickly and completely in water. The dissociated ions can form dense hydrogen bonding interactions with water molecules, giving SMAS excellent water solubility (no obvious upper solubility limit at room temperature). This also ensures that its copolymerization products (e.g., SMAS-AM copolymers) do not agglomerate or precipitate in water—this is a prerequisite for its use as a “water-based drilling fluid additive” and “water-soluble polymer monomer”.
- Salt Resistance and Environmental Stability: The sulfonate group (-SO₃⁻) has high negative charge density and strong ionic bond strength, and is unlikely to combine with metal ions such as Ca²⁺ and Mg²⁺ in oilfield formation water to form precipitates (unlike the carboxyl group -COOH, which easily chelates with metal ions and causes polymer salting-out). This salt resistance allows SMAS-based polymers to maintain stable performance in high-salinity environments (e.g., 12×10⁴ mg/L salinity in the Bohai Oilfield), avoiding sudden viscosity loss or functional failure—this is the irreplaceable key to its application in “oilfield high-salt oil displacement agents” and “oily wastewater flocculants”.
- High Charge Density and Function Transfer: -SO₃Na endows SMAS molecules with strong anionic properties. When SMAS copolymerizes with other monomers (e.g., acrylamide (AM), acrylic acid (AA)), it transfers this “high charge density” to the copolymer. For example: in oilfield oil displacement agents, high charge density causes polymer chains to expand in water due to electrostatic repulsion, significantly increasing the viscosity of the aqueous solution (enhancing mobility control capabilities); in oily wastewater treatment, high charge density can adsorb negatively charged crude oil droplets and suspended particles in wastewater, achieving efficient flocculation through “charge neutralization + bridging effect”.
2. Carbon-Carbon Double Bond (C=C): Determines “Polymerization Reactivity and Functionalization Potential”
The carbon-carbon double bond is an unsaturated reactive site in the SMAS molecule, endowing it with the core reaction characteristics of “polymerizable, copolymerizable, and customizable”—this is the fundamental reason why it can serve as a “functional monomer” to form polymer materials. SMAS itself is rarely used alone, and its application value almost entirely depends on functional polymers formed by copolymerization with other monomers via the carbon-carbon double bond:
- High Free Radical Polymerization Reactivity: The π bond in C=C has high electron cloud density and is easily attacked by free radicals (e.g., ·OSO₃⁻ generated by persulfate decomposition), undergoing free radical polymerization or copolymerization reactions. For example: SMAS can copolymerize with monomers such as acrylamide (AM) and 2-acrylamido-2-methylpropanesulfonic acid (AMPS) to form linear or slightly branched polymer chains—this polymerization ability allows SMAS to be converted from a “small-molecule monomer” to a “polymer with specific functions” (e.g., drilling fluid fluid loss reducer, oil displacement agent).
- Structural Adjustability and Functional Customization: The methyl group (-CH₃) adjacent to the carbon-carbon double bond produces slight steric hindrance, which can regulate the rate of copolymerization reactions and the structure of polymer chains: on the one hand, steric hindrance can reduce the degree of polymer branching, making the chain structure more linear and enhancing the mechanical strength of the product (e.g., the shear resistance of SMAS-AM copolymers, which is suitable for the pipeline shear environment during oilfield oil displacement agent injection); on the other hand, by adjusting the copolymerization ratio of SMAS to other monomers (e.g., SMAS:AM:AMPS = 2:5:3), the viscosity, charge density, and other properties of the copolymer can be precisely controlled to match the needs of different scenarios (e.g., low-permeability reservoir completion fluids require low charge density, while high-salt oil displacement agents require high salt resistance).
Conclusion: “Synergistic Effect” of the Two Functional Groups
The chemical properties and application value of Sodium Methallyl Sulfonate (SMAS) result from the complementary synergy of the two functional groups:
- The sodium sulfonate group (-SO₃Na) is responsible for “basic adaptability”—ensuring that SMAS and its derivatives can exist stably (dissolved, non-failing) in aqueous/high-salt environments;
- The carbon-carbon double bond (C=C) is responsible for “functional realization”—transferring the properties of SMAS (salt resistance, high charge) to the copolymer through polymerization reactions, and endowing the polymer with customizable structure and performance.
Neither group can be omitted: without -SO₃Na, SMAS would lose water solubility and salt resistance, making it unable to adapt to aqueous systems; without C=C, SMAS cannot polymerize to form polymer materials and can only exist as a small molecule, losing its application value in oilfields, polymer synthesis, and other fields.